University of Florida Study: What Makes a Smell Bad?
A team from the University of Florida discovered two neural pathways from the amygdala to the ventral striatum that determine whether an odor is perceived as a threat or a reminder. The discovery reveals key mechanisms of olfactory avoidance.

Imagine placing a huge garbage container next to your living room: the stench would fill every corner and burn itself into your memory. That foul odor immediately sparks rejection and is certainly never associated with anything pleasant.
Researchers at UF Health have just revealed how our brain decides that a smell is unpleasant. Thanks to advanced techniques in mice, they mapped two parallel neural pathways that connect the basolateral amygdala (BLA) to the ventral striatum, determining whether a smell triggers aversion or fear.
Two Routes to the Same Emotion
Imagine your brain as a city and smells as taxis arriving at emotion control central: the basolateral amygdala (BLA). Two control teams with different genetic uniforms operate there—Drd1+ and Drd2+ neurons—which, although they start from the same place, take separate routes to the ventral striatum, the region that decides whether a scent prompts an immediate escape or gets permanently stored in memory.
The Drd1+ neurons take the highway leading to the nucleus accumbens (NAc), well-known for its role in reward and, in this case, in immediate avoidance. Like a radar, they detect a foul smell and launch the signal: “Get out of here!”
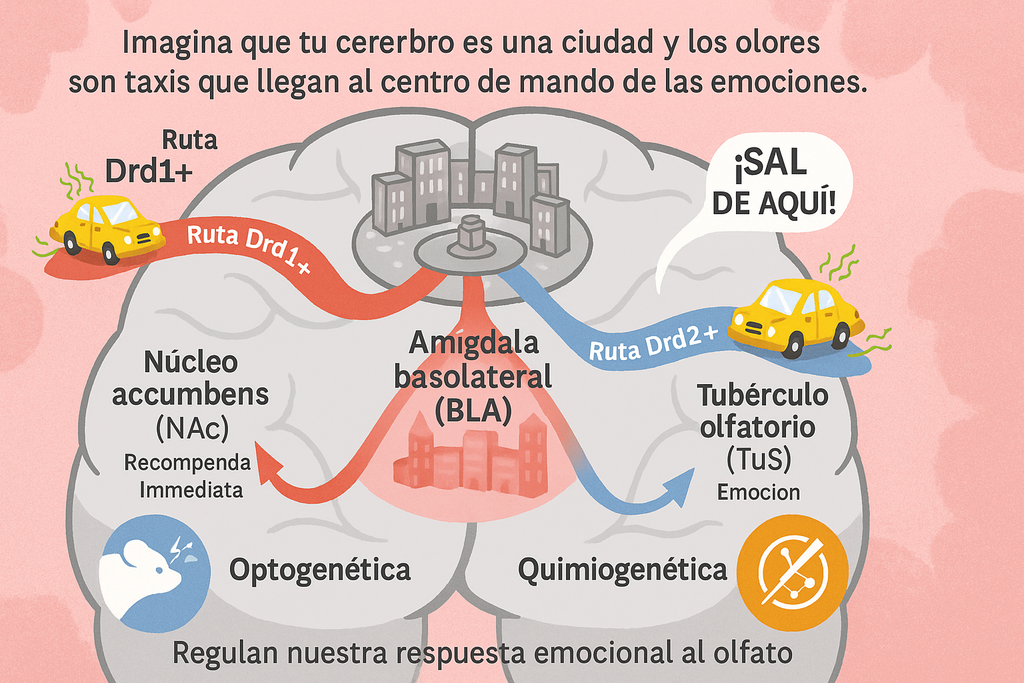
On the other hand, Drd2+ neurons take the road to the olfactory tubercle (TuS), which specializes in processing the chemical information of smells and linking it to learned emotions. When they kick into action, they not only provoke an immediate escape but also reinforce the memory of that smell as something to fear.
Armed with this information, they showed that each “team” sends highly specific and distinct instructions to the ventral striatum, shaping our reaction to olfactory stimuli. But how can their function be tested in real time? That’s where two tricks come in:
- Optogenetics: Using pulses of blue light, scientists “switched on” Drd1+ terminals in the NAc. The result was striking: mice avoided the lit-up area by almost 50% more than usual.
- Chemogenetics: They installed “chemical keys” (DREADD receptors) that, when exposed to a harmless compound, “turned off” that same route. Without it, the mice could no longer associate a mild electric shock with a specific odor.
When they repeated the experiment with the Drd2+→TuS pathway, both the immediate escape and the ability to learn to fear the smell were affected. In short, there’s no single “bad smell”: two parallel highways, working in sync, regulate our emotional response to scent.
From Science to Olfactory Well-being
This discovery isn't just a neurological curiosity—it opens the door to practical applications in mental and sensory health. Imagine your anxiety alarms are linked to a “garbage sensor” in your brain: a common odor could trigger panic in people with PTSD or olfactory hypersensitivity. If we learn how to temporarily “disconnect” the Drd1+ or Drd2+ pathways, those alarms might be silenced, restoring calm.
But the nose has even more tricks up its sleeve. What if, instead of switching them off, we activate those same pathways in patients who have lost their appetite? We could reignite the pleasure of food aromas and awaken hunger like flipping on the lights for a culinary party. After all, a happy nose can do wonders for a healthy body!

And this is just the beginning. The team’s next step is to find out how dopamine—the brain’s “traffic manager”—regulates these emotional pathways. They also plan to explore every corner of the NAc and TuS to create even more detailed maps of their detours and shortcuts. The ultimate goal is to design precision therapies: surgical interventions in neural traffic without short-circuiting the rest of the olfactory network.
As Wesson aptly puts it, “Understanding how the environment shapes our emotions is the key to becoming happier and healthier.” Who would have thought: behind a simple scent lies an entire web of brain highways, ready to be discovered… and to teach us to smell life differently.
News References
-Nature. Targeting negative emotional states via parallel genetically distinct pathways from the basolateral amygdala to ventral striatum subregions. (2025).
-UF Health. Study: What makes a smell bad? (2025).




